Targeting the gene Foxo1 may offer an early treatment approach for hereditary lymphedema, USF Health preclinical study reports

Principal investigator Ying Yang, PhD, is an assistant professor of molecular pharmacology and physiology in the USF Health Morsani College of Medicine and a member of the college’s Heart Institute. | Photo by Allison Long, USF Health Communications
Tampa, FL (Aug. 9, 2021) — A University of South Florida (USF Health) preclinical study unexpectedly identified the gene Foxo1 as a potential treatment target for hereditary lymphedema. The research, published July 15 in The Journal of Clinical Investigation, was done with colleagues from Tulane University and the University of Missouri.
Lymphedema — a chronic condition in which lymphatic (lymph) fluid accumulates in soft tissue under the skin, usually in the arms and legs — causes minor to painfully disfiguring swelling. Primary, or hereditary, lymphedema is rare, present at birth and caused in part by genetic mutations that regulate normal lymphatic valve development. Secondary, or acquired, lymphedema is caused by damage to the lymphatic system from surgery, radiation therapy, trauma, or parasitic infection. In the U.S., lymphedema most commonly affects breast cancer patients, with prevalence ranging from 10 to 40% after lymph node removal and radiation therapy.
While lymphedema can be managed with massage and compression garments, no treatment exists to address its underlying cause: the build-up of fluid that eventually backs up in the lymph system like an overflowing sink with a blocked drain. This stagnant lymph triggers an inflammatory response that can induce connective and fatty tissue to form and harden the skin, restricting movement and increasing the risk of recurrent infections.
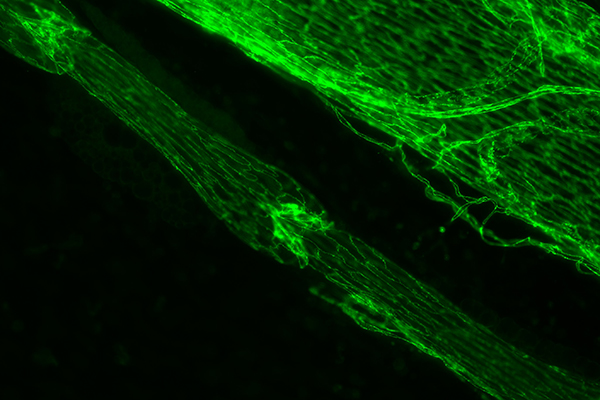
Green immunostained image of a lymphatic vessel with one valve in the center and another in the top left corner. To the upper right of the lymph vessel is a large vein. | Photo courtesy of Joshua Scallan, PhD.
“The later fibrosis stage of lymphedema cannot be massaged away,” said study principal investigator Ying Yang, PhD, assistant professor of molecular pharmacology and physiology at the USF Health Morsani College of Medicine. “Targeting lymph valves early in the disease is one critical aspect in identifying an effective treatment for lymphedema. If the disease progresses too far, it’s difficult to reverse.”
Valve loss or dysfunction that disrupts the flow of lymph fluid is strongly associated with lymphedema in patients. But no one has discovered whether new valves can be grown or if defective ones can be fixed.
The USF Health-led study shows that both are possible.
Dr. Yang’s group hypothesized that the protein encoded by the gene Foxo1 plays a key role in lymph valve formation based on an earlier USF Health discovery of cell signaling processes controlling formation of lymph valves. The researchers showed that deleting a single gene — lymphatic vessel-specific Foxo1 — promoted the growth of markedly more valves in both young postnatal mice and adult mice than in control littermates without Foxo1 deletion. Furthermore, deleting Foxo1 in a mouse model mimicking human lymphedema-distichiasis syndrome fully restored the both the number of valves and valve function.
“It was exciting to see that Foxo1 is the only gene so far reported that, when deleted, induces more lymphatic valves to form, instead of inhibiting valve growth,” said Dr. Yang, a member of the USF Health Heart Institute. “We actually reversed valve loss and repaired the structure and function of defective valves in a genetic mutation model of lymphedema…That type of discovery makes a study clinically relevant.”
The lymphatic circulatory system – a parallel of the blood vessel circulatory system – helps maintain healthy fluid balance in the body by collecting and controlling the flow of extra lymph fluid that leaks from tissue. This complex network propels watery lymph fluid carrying proteins, nutrients and toxin-destroying immune cells through the body in one direction before returning the fluid to circulating blood. Small valves inside lymph vessels open and close in response to force exerted by the lymph fluid, moving it forward and preventing backward flow into tissues.

Dr. Yang in her lab where she researches lymphedema, which in the U.S. most commonly occurs in some breast cancer patients after lymph node removal and radiation therapy. Some of the research is light sensitive and must be conducted in near darkness. | Photo by Allison Long, USF Health Communications
Among the key study findings:
- The protein FOXO1 (encoded by gene Foxo1) inhibits lymph valves from developing by suppressing many genes, which collectively contribute to the multi-step process of making a mature valve. FOXO1 behaves like a brake on a set of valve-forming genes, Dr. Yang said. “Once the brake is removed, all those genes can now be expressed so that new valves can successfully grow.”
- Inactivation (knockout) of Foxo1 in lymphatic endothelial cells (LEC) of young postnatal mice promoted valve formation at multiple stages. Likewise, deleting LEC-specific Foxo1 in adult mice also increased valve formation, compared to control mice without the gene knockout.
- A mouse model of lymphedema-distichiasis syndrome had 50% fewer lymphatic valves and the remaining valves closed abnormally and exhibited fluid backflow. But when Foxo1 was deleted, the number of valves increased to the same levels as those in healthy control mice and the structure of defective valves was restored to normal. Further analysis showed that the loss of Foxo1 also significantly improved valve function in this mouse model of human primary lymphedema disease.
This study was supported by grants from the National Heart, Lung, and Blood Institute, a part of the National Institutes of Health. USF Health’s Joshua Scallan, PhD, was the lead author.