The microbiota (community of bacteria, fungi and other microbes) and microbiome (interplay between the microbiota and the environment) offer a novel paradigm for modern transdisciplinary science. In fact, we now recognize that health of the biosphere, including human health, relies on a more comprehensive, integrated understanding of these communities of microbes.
With that in mind, USF has launched a university-wide initiative to create a hub for research into microbiota and microbiomes. More about that later. First, let’s review some of what we’ve learned about the microbiome to help frame why this emerging area of research is so vital to the future of medicine and other disciplines.
Microbiome Research
Microbiome research encompasses the study of human and animal health and nutrition, as well as the wider environment that includes marine and soil microbiota communities. The concept of bacterial populations rather than single pathogens has linked medicine and public health with computer science, data analytics, artificial intelligence and engineering. In this new approach, individuals are considered ecological systems in a process of co-evolution between human hosts and bacteria and maintaining health encompasses management, restoration and sustainability.
Microbiomes, therefore, have major implications when it comes to intervening in environmental and health processes. They are also a fine illustration how technological progress shapes science.
While the importance of the microbiome in the lungs and airways, skin and vagina is increasingly recognized, the gut microbiome has received the most attention to date. It is now clear that an imbalance in gut microbes (dysbiosis) is linked to several diseases, including metabolic disorders such as obesity and diabetes, cardiovascular diseases and stroke, inflammatory bowel diseases and colon cancer, non-alcoholic fatty liver disease, and chronic respiratory disorders such as asthma. Numerous studies have also emphasized the importance of the microbiome in perinatology, potentially shaping the future of newborns and influencing the post-partum health outcomes of mothers. Some studies suggest that gut dysbiosis influences neurodegenerative conditions (Parkinson’s and Alzheimer’s disease) and possibly psychiatric disorders such as depression. The impact of the microbiota in cancer patients is doubtless one of the most stimulating areas of research for developing new therapies. Not only has gut dysbiosis been demonstrated in various forms of cancer, but microbiome composition has been shown to interfere with the efficacy of chemotherapy and immunotherapy.
The interplay between the gut and lung microbiomes and infectious diseases – such as in Clostridium difficile or norovirus infection and malaria – is also notable. Moreover, the role played by the insect gut microbiota in mosquitoes’ ability to transmit viruses such as Zika and dengue opens a fascinating new avenue for investigation, raising the possibility of future strategies in this field. Infectious disease prevention through vaccination will also likely emerge as another major research area. Indeed, understanding the role of host microbiota is critical for optimizing the immune response to microbial vaccines. Variability in vaccine-induced immunity is due to the environment, human genetics, maturation of the immune system according to age and sex, and socioeconomic conditions. However, the diversity and quality of microbiota in vaccine studies has so far been neglected, despite evidence of its impact.
The Impact on Health and Environment
Overall, the number of medical disorders potentially associated with microbiome imbalance continues to rise. Anthropological investigations might even provide a clue about the evolution of the microbiome in different contexts. Also, importantly, the impact of the microbiome on metabolism, pharmacodynamics and overall efficacy of many drugs has emerged as a most important challenge for the future. Along the same lines, the microbiome might mediate some toxic effects of environmental stress on learning and development, for example, and also in alcohol and substance abuse and co-occurring disorders.
Evidence indicates we can act on the microbiome to influence the clinical characteristics of patients. Probiotics have been widely used for a century and, although their efficacy is still a matter of debate, they may benefit at least some patients. Transplanting gut microbiota to germ-free (gnotobiotic) mice represents a major step forward in establishing causal relationships between dysbiosis and some clinical conditions. In humans, the efficacy of fecal microbiota transplantation has been clearly established for the treatment of Clostridium difficile infection and is currently being evaluated for several other medical conditions. Nanotechnology can also provide a novel approach to microbiome intervention. Finally, some evidence shows that determining bacterial and/or metabolite signatures may help predict susceptibility to disease and response to therapy. In fact, microbiome analysis can be integrated into prospective deep longitudinal molecular and physiological profiling studies and provide important tools for precision health. Thus, the microbiome can now be considered part of a precision medicine strategy.
Besides human health, microbiomes also impact environmental microbiology, including in relation to climate change and food insecurity. Quantifying bacteria and viruses that are strongly associated with the GI tracts of specific host species or groups (referred to as “microbial source tracking”) can be very helpful in determining sources of fecal contamination in environmental waters and food, including microalgae. Thus, it comes as no surprise that microbiome interest is currently a major focus of research worldwide for both academics and pharmaceutical companies, whether for environmental or health purposes. The global human microbiome market is expected to reach $1.2 billion in 2025, up from nearly $260 million in 2017.
The Challenges Ahead
- Microbiota taxonomy is still evolving, with much yet to be discovered. Studies have mainly relied on 16S RNA sequencing, whereas whole metagenome sequencing is required to accurately describe bacterial populations. Sophisticated approaches for culturing microbes (referred to as culturomics) may also further increase our understanding.
- The microbiota includes not only bacteria but also viruses (mostly phages), fungi, archaea and parasites. Focusing predominately on bacteria may have resulted in erroneous interpretations in studies that overlooked other microbes.
- Geographical differences, involving environment, nutrition and human genetics, may greatly influence the results of these studies.
- Most research has been descriptive. Composition of the gut microbiome associates with a wide range of human diseases, but the mechanisms underpinning these associations are not well understood. There is a genuine need for functional studies; indeed, it has become increasingly clear that different bacterial “signatures” may share the same functional impact through their secreted metabolites. To shift toward a mechanistic understanding, we need to use an integrated approach and identify functions encoded in the gut microbiome linked with either multiple or specific diseases. Fecal microbiota transplantation in various animal models and in-depth immunological investigations will be key to achieving this objective as will the ability to mimic bacterial diversity and metabolite production in bioreactors.
- The real efficacy of medical procedures, including probiotics or fecal microbiota transplantation, has not been clearly demonstrated.
These challenges can only be overcome by combining scientific and medical expertise. Attaining success requires translational efforts including basic and technological science and top-quality clinical research, as well as robust infrastructures and equipment comprising omics (genomics, proteomics and metabolomics) and germ-free animal facilities. Incorporating disciplines such as synthetic biology, chemistry, engineering and nanotechnology can greatly improve this approach. Product development and strong interaction with industry players and venture capital is another key element. Thus, data analysis capabilities are critical for any major microbiome research initiative.
Our Vision at USF
Overall, this initiative is about a new way of organizing science based on fluid programs across colleges and departments — a move away from the standard model of separate departments. The University of South Florida is poised to be on the crest of this disruptive and innovative sea change, a scientific and medical revolution that will bring global recognition while opening new streams of funding not only for the USF Health Morsani College of Medicine, but across the entire university.

Visit the new USF Initiative on Microbiomes website at health.usf.edu/medicine/microbiome.
Several high-level scientists are already working on the microbiome at USF and Moffitt Cancer Center, and several have either initiated small-scale projects or shown interest in developing larger-scale original projects. Yet, many work in isolation and coordinating microbiome research efforts are needed to succeed. For this reason, we created the Microbiome Club as a first step of this initiative to identify, engage and pool the work of talented investigators and to share viewpoints and needs. This is also the aim of the Microbiome Awards, which will provide seed funding for the early phases of novel, interdisciplinary and transdisciplinary projects involving several departments and colleges. These projects will also serve as a basis for grant applications. Thanks to a newly created web site we will develop an effective strategy for communicating and sharing data with an online forum, joint workshops, mentoring, and support for grant applications.
We want to go beyond these first steps and advance to a truly transdisciplinary approach, with programs spanning the various colleges and departments. To grow the initiative we must offer mentoring for junior investigators and work closely with the various colleges and undergraduate programs to provide effective training for early career scientists.
Several studies emphasize the value of comparing results on how the microbiome affects the environment and health in different geographical areas. That means we need to expand international collaborations. Clearly our goal is to foster innovation that translates research into care, and this implies developing close academic and industry partnerships. We need integrated analysis not only of the ecological and environmental impacts on microbiomes, but of the research interests in cardiovascular, neurodegenerative, psychiatric disorders, and cancer and infectious diseases.
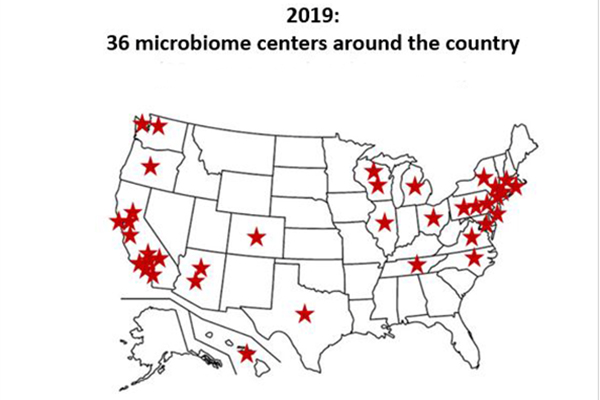
Transdisciplinary collaborations will be at the heart of our success. Microbiome research represents a new challenge for most institutions and, despite currently lagging behind many universities and centers, USF can contribute to breakthrough solutions by developing sound projects and generating significant return on investment. This initiative will also enable USF and Moffitt to pool the expertise of their colleges, departments and centers, which can provide a solid basis for tackling other research challenges to come. It will strengthen cooperation between universities in Florida (such as with the University of Florida) and in the United States. Microbiome research is fiercely competitive; at least 30 centers or initiatives are dedicated to microbiome studies nationwide. With this in mind, USF has organized joint workshops with the University of Florida and Moffitt. Finally, we believe that building a robust microbiome initiative offers an excellent opportunity to help foster entrepreneurship and academic-industry partnerships in the Tampa Bay region, Florida, the U.S. and the world.
Christian Brechot, MD, PhD
Senior Associate Dean for Research in Global Affairs, MCOM
Associate Vice President, International Partnerships and Innovation
Professor, Department of Internal Medicine