Targeting VE- cadherin signaling pathways holds promise for treating lymphedema’s debilitative swelling, USF-led preclinical research suggests
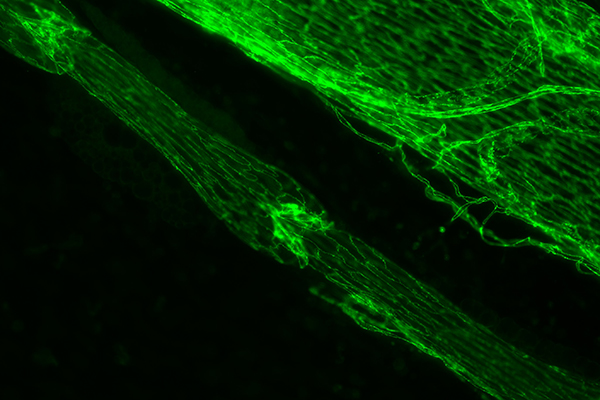
Green immunostained image of a lymphatic vessel with one valve in the center and another in the top left corner. To the upper right of the lymph vessel is a large vein. | Photo courtesy of Joshua Scallan
TAMPA, Fla. (Aug. 27, 2019) — Lymphedema, resulting from a damaged lymphatic system, can be a debilitating disease in which excess protein-rich fluid (lymph) collects in soft tissues and causes swelling — most often in the arms or legs. Symptom severity varies, but the chronic swelling can lead to pain, thickened skin, disfigurement, loss of mobility in affected limbs, and recurrent infections.
While lymphedema can be managed with massage and compression garments or electronic sleeve-like pumps, no treatment exists to address its underlying cause: the abnormal build-up of lymph fluid that expands tissue like a water-logged sponge.
Now, a mouse model study led by the University of South Florida (USF Health) Morsani College of Medicine has identified new cellular processes controlling development of the small valves inside lymphatic vessels, which prevent lymph fluid from flowing the wrong way back into tissues. The one-way valves work with muscles to help propel lymph fluid through the body and regulate flow. The new findings suggest that targeting signaling pathways involved in creating and maintaining lymphatic valves may one day be a viable therapy for patients coping with lymphedema.
The study was published online Aug. 27 in Cell Reports.

Joshua Scallan, PhD, assistant professor of molecular pharmacology and physiology at USF Health Morsani College of Medicine, focuses on the molecular and cellular processes of lymphatic vessels. The lymphatic system parallels the blood vessel circulatory system, but has been studied less extensively.
“We knew that lymph flow was required for the valves to form and function throughout life, but we did not know how the endothelial cells that make up the inner lining of lymphatic vessels can ‘sense’ the flow,” said senior author Joshua Scallan, PhD, assistant professor in the USF Health Department of Molecular Pharmacology and Physiology. “This study is the first to identify signaling pathways by which cells sense and respond to the mechanics of flow to keep the valves operating effectively — so that lymph fluid keeps moving forward.”
The lymphatic circulatory system – a parallel system to the blood vessels – is an extensive drainage network that acts as a conduit for immune cells (such as lymphocytes) and helps protect the body against infections. One of its primary jobs is to remove extra fluid (mostly water containing proteins, lipids, and other substances) that continuously leaks from tiny blood vessels into surrounding tissues just under the skin, Dr. Scallan said.
The lymph vessels, which carry lymph around the body, collects the leaked fluid (as much as 12 liters a day) and transports it away from the tissues. This lymph fluid is checked and filtered for bacteria, viruses and other harmful substances by lymph nodes clustered in various areas of the body, and eventually returned to the blood circulatory system through veins in the neck. If forward lymph flow is blocked or impaired, fluid does not drain from the tissues and results in the disease known as lymphedema.
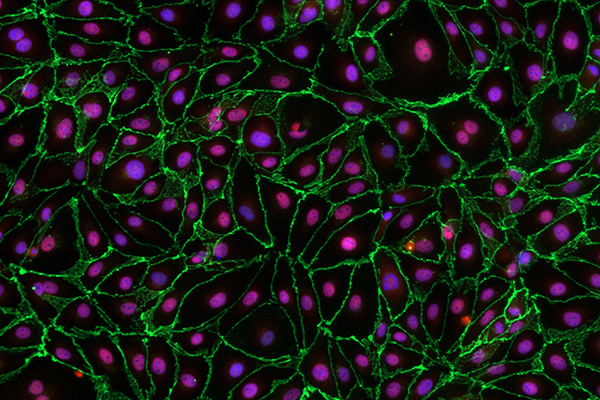
Microscopic image of endothelial cells lining the lymphatic vessels. The protein VE-cadherin (stained green) is needed for lymphatic valves to form, mature and maintain the normal movement of lymph fluid. | Photo courtesy of Joshua Scallan
In a series of experiments, the researchers used a “conditional knockout” mouse model, developed in Dr. Scallan’s laboratory, in which production of the junction protein VE-cadherin was inactivated in lymphatic vessels. The gene for VE-cadherin, located where neighboring cells lining lymph vessels connect, was deleted in mice both before and after birth.
Among key findings of the preclinical study detailed in Cell Reports:
- Deletion of VE-cadherin prevented lymphatic valves from forming in the embryonic mice and caused disintegration of valves already developed in the postnatal mice. This indicates that the protein is required for lymphatic valves to form, mature and maintain the normal movement of lymph fluid away from tissues.
- Stimulating two different signaling pathways dependent upon VE-cadherin activation — β-catenin and AKT – partially rescued the loss of valves.
- The AKT signaling pathway was shown to promote the growth of new valves in normal, healthy mice.
“Our data explain how fluid forces at the lymphatic endothelial cell membrane regulate genes to control valve formation and maintenance,” Dr. Scallan and his fellow study authors concluded. “Future studies are needed to investigate the AKT signaling pathway to identify therapeutic targets that may safely enhance valve formation in lymphedema patients.”
Millions of people worldwide suffer from lymphedema, predominantly in tropical and subtropical regions where filariasis (a parasitic infection) is common. In the U.S. and most other developed countries, acquired (secondary) lymphedema is most commonly caused by breast cancer treatment: surgical removal of lymph nodes or radiation therapy. Hereditary (primary) lymphedema, caused by defective lymph vessels at birth, is rare.
USF Health researchers worked with colleagues at the Oklahoma Medical Research Foundation. The study was supported by grants from the National Heart, Lung and Blood Institute, National Institutes of Health.
-Photos by Allison Long, USF Health Communications and Marketing