In the laboratory and the clinic, Dr. Thomas McDonald focuses on inherited heart diseases that can lead to potentially deadly heart rhythm disturbances
Sudden cardiac death most often makes the news when athletes in peak physical condition collapse and die while exercising or competing. This spring, Zeke Upshaw, 26, a basketball player for the Grand Rapids Drive, a G-league affiliate of the Detroit Pistons, collapsed face-down on home court during the final minute of a game and later died at the hospital. A medical examiner ruled that he had suffered sudden cardiac death.
“Sudden cardiac death is when someone, usually otherwise healthy and often young, tragically drops dead – without any warning,” said Thomas V. McDonald, MD, a professor in the USF Health Department of Cardiovascular Sciences.
Most of the 200,000 to 450,000 sudden cardiac deaths each year in United States are caused by heart rhythm disturbances provoked by certain strenuous activities, prescription medications, recreational drugs, or other triggers. “Sometimes it just happens in your sleep. The most severe and earliest form would be sudden infant death, or SIDS,” Dr. McDonald said.
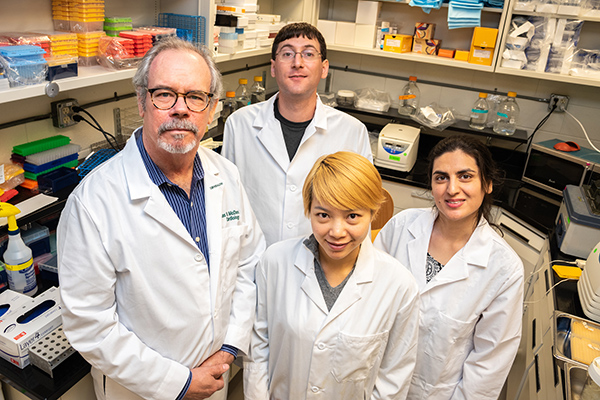
Physician-scientist Thomas McDonald, MD, a professor in the USF Health Department of Cardiovascular Sciences and member of the USF Health Heart Institute, with his laboratory team.
Dr. McDonald was recruited to the USF Health Heart Institute in October 2017 from Albert Einstein College of Medicine in New York City, where he was a professor of both cardiology and molecular pharmacology. He also co-directed the thriving Montefiore-Einstein Clinic for CardioGenetics, the first such interdisciplinary clinic in metropolitan New York for families at risk of sudden cardiac death from arrhythmias.
At USF Health his laboratory continues to focus on the fundamental causes of heart conditions passed from one generation to the next — and what can be done to help prevent disease and its consequences. The hereditary conditions he studies include those affecting the heart’s electrical system to cause arrhythmias, like long QT syndrome and Brugada syndrome, and those affecting heart muscle, such as hypertrophic cardiomyopathy and dilated cardiomyopathy. While rare, these conditions can substantially increase an individual’s risk for sudden cardiac death and devastate families.
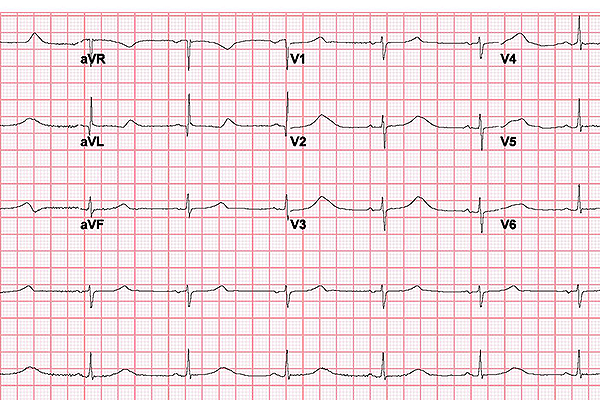
In long QT syndrome, the heart takes longer than normal to recharge between beats. This electrical disturbance, called a prolonged QT interval, can often be seen on an electrocardiogram (ECG) like the one pictured here.
Dr. McDonald has also started a USF Health Cardiogenetics Clinic, modeled after the Montefiore-Einstein center he co-founded, to evaluate and treat families in which members succumb to unexplained sudden cardiac death or SIDS, or where suspicion of an underlying, hereditary heart rhythm disturbance exists.
His work bridging the laboratory and clinic has implications for a much larger population than people with relatively rare inherited cardiac disorders. Dr. McDonald points to growing evidence of the interplay between genetics and environmental factors like diet, exercise and stress.
“By studying these rare or uncommon cardiac diseases,” he said, “we may uncover more generalizable biochemical pathways that could be influenced to harm the heart given the wrong environment — even in genetically unaffected families.”
![]() Studying uncommon (inherited) heart diseases to gain better insight into more common ones.
Studying uncommon (inherited) heart diseases to gain better insight into more common ones.

Dr. McDonald lifts a container including pluripotent stem cells from storage in liquid oxygen. Alexander Bertalovitz, PhD, (right) an assistant professor of cardiovascular sciences who helps manage the cardiogenetics laboratory, followed Dr. McDonald to USF Health from Albert Einstein College of Medicine in New York City.
Pinpointing the meaning of genetic variants of “unknown significance”
Dr. McDonald analyzes genetic changes, or mutations, which may lead to malfunctioning of ion channels that create electrical signals in the heart.
His team has spent the last few years characterizing the function of 1,000 different mutations found in cardiac ion channel genes associated with hereditary rhythm conditions such as long QT syndrome and Brugada syndrome. The researchers recreate the genetic variations in a cellular model and use automated electrophysiology techniques to analyze how the mutations affect the ion channel’s ability to correctly generate each heartbeat. All these variations have been cited in published scientific literature; however, it is still largely unclear which ones truly increase the risk of abnormal rhythms leading to palpitations, seizures, fainting or sudden death – and which are benign.
The research project is supported by a five-year, $1.7 million R01 grant from the NIH’s National Health, Lung and Blood Institute.
“Our ultimate goal is to work with other investigators to create a NIH-curated public database that physicians and genetic counselors could access to find out whether a genetic variant is likely, or unlikely, to cause a potentially life-threatening heart rhythm disturbance in a patient or their family members,” Dr. McDonald said.
As genetic testing is becomes more common, a growing challenge is that lab reports of people referred for DNA sequence testing often come back listing many “variants of unknown significance,” Dr. McDonald said. “That drives physicians and patients crazy because they don’t know what that means… what do they do with that information?”

Dr. McDonald with Jiajia Yang, a PhD student in the Department of Molecular Pharmacology and Physiology.
An important step toward improving the guidance that doctors offer individuals with inherited heart disorders would be the ability to more precisely distinguish between disease-causing mutations and mutations with little or no harmful physiological effects through a resource like a scientifically validated database, he added.
Recommended treatment options for long QT are life-long and vary, including regular cardiac monitoring, taking medication such as beta blockers, restricting strenuous sports activities, or sometimes implanting pacemakers or defibrillators to help control abnormal heartbeats. So, for example, if DNA testing of a child or young adult revealed a long QT genetic variation characterized as having little risk of leading to sudden cardiac death, prescribing beta blockers and routine cardiac monitoring might be the best preventive therapy – avoiding the long-term management and small, but real, lifetime risk of complications from an implantable device.
![]() Dr. McDonald comments on the focus of his laboratory’s research on genetic variations.
Dr. McDonald comments on the focus of his laboratory’s research on genetic variations.
Opening Tampa Bay region’s first CardioGenetics Clinic
The twice-monthly Cardiogenetics Clinic, which opened in March, is held at USF Health Cardiology’s Armenia Avenue location. The new clinic is staffed by a team with the expertise to address the diverse medical, psychological, social and ethical issues arising when evaluating genetic heart conditions that predispose patients to sudden cardiac death.

Dr. McDonald — with certified genetic counselor Melissa Racobaldo (far left) and clinical geneticist Christopher Griffith, MD — leads a comprehensive discussion of family medical history with a patient and his mother referred to the USF Health Cardiogenetics Clinic.
“When I arrived there was no formal cardiogenetics program in the greater metropolitan area of Tampa Bay where 4 million people live — so the prospect of building one from scratch was very attractive,” said Dr. McDonald, who specializes in adult cardiology. He leads the clinic working with USF Health faculty members Christopher Griffith, MD, assistant professor of pediatrics and a clinical geneticist; and Melissa Racobaldo, a genetic counselor; as well as Gary Stapleton, MD, a pediatric interventional cardiologist from Johns Hopkins All Children’s Hospital. USF College of Public Health students specializing in genetic counseling are expected to join the clinic in coming months.
Many with congenital cardiac conditions have no signs or symptoms. Patients and their families referred to the clinic typically have experienced a history of arrhythmias or other cardiac events, or suffered the unexpected death of a loved one.
During the initial visit, families meet with team members for a cardiac history and examination, review of medical records and/or autopsy reports, and baseline tests that include an electrocardiogram and echocardiogram. Based on the family’s medical history, a tree-like chart known as the DNA pedigree is created to identify familial genetic patterns and sudden unexpected deaths linked to cardiac disorders. “The most important genetic test is still a complete family history,” Dr. McDonald said.
DNA testing is usually only recommended when the team discerns that the pattern of cardiac-related events is highly likely to be genetic rather than environmental. For instance, a family history indicating that a few relatives died from heart disease in their 80s might be considered environmental.
If a mutation is found in one of the genes known to be associated with a dangerous cardiac arrhythmia, then the first (affected) patient who got tested receives immediate counseling by a cardiologist, and genetic testing and counseling will be offered to all at-risk relatives. Different heart rhythm genetic mutations have different effects on ion channels, so individualized remedies are required.
“Genetics is still quite complex to most people, so we try to make our explanations understandable and not so scary,” said Dr. McDonald, who has co-authored several articles on how patients are affected by cardiogenetic testing, including in the journals Qualitative Health Research and Personalized Medicine.
“Our dominant message is ‘we’re here to provide information, which gives you knowledge, and knowledge gives you power to manage your life and to help the next generation.”
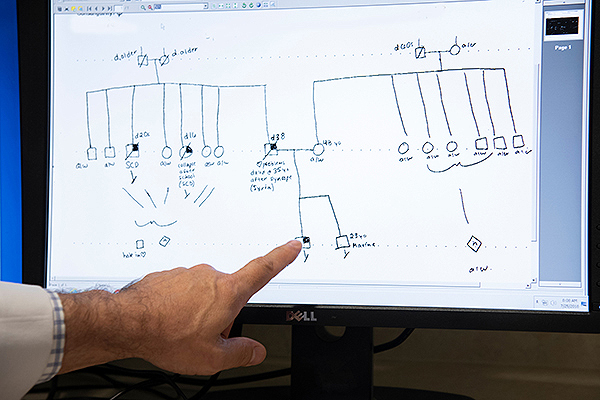
A pedigree, which depicts the relationship between individuals and relevant facts about their medical histories, can be used to help understand the transmission of genes within the family. Dr. McDonald points to a square indicating the presence of a particular genetic trait in a male.
The Cardiogenetics Clinic will offer patients access to the latest clinical trials for new drugs or devices. Dr. McDonald was recently named USF site lead investigator for a world-wide Phase 3 study testing the effect on walking endurance of an investigational medication for patients with dilated cardiomyopathy caused by a rare genetic mutation. This form of heart disease, in which inadequate pumping of blood causes the heart to become weaker, can lead to heart failure.
“Heart in a dish” as a drug screening tool
Interested in drug discovery for inherited heart diseases lacking effective medications, Dr. McDonald’s lab has begun collecting blood cells from USF Health cardiomyopathy patients who provide informed consent.
The adult blood cells can be genetically reprogrammed into induced pluripotent stem cells (iPSCs) with the potential to develop into any cell type in the body, including heart cells. The goal is to model the early stages of inherited heart disease with patient-specific cells grown in a petri dish, working out at a molecular level how the disease does its damage to heart muscle.
![]() On the horizon: Modeling inherited heart diseases using pluripotent stem cells
On the horizon: Modeling inherited heart diseases using pluripotent stem cells

Dr. McDonald and Maliheh Najari Beidokhti, PhD, a postdoctoral associate in the Department of Cardiovascular Sciences.
“Once you do that,” Dr. McDonald said, “you can use the ‘heart disease in a cell culture dish’ to screen any number of drugs or chemical compounds for their potential therapeutic benefit.”
Dr. McDonald is also collaborating with colleagues in the USF Health Department of Neurology to look at rare genetic mutations for nervous system diseases, such as certain types of muscular dystrophy and ataxias, which can lead to severe heart damage,
Dr. McDonald received his bachelor’s degree in zoology from USF in 1977 and MD degree from the University of Florida. He completed a residency in medicine and research fellowship in cardiology at Columbia-Presbyterian Medical Center in New York City. At Stanford University School of Medicine, he conducted fellowships in clinical cardiology and interventional cardiology, as well as a postdoctoral research fellowship. He spent 22 years as a faculty member at Albert Einstein College of Medicine before joining the USF Health Morsani College of Medicine last fall.
Continuously funded throughout his career by the NIH or the American Heart Association (AHA), Dr. McDonald has authored more than 70 peer-reviewed publications. Among his many high-impact papers was a 2013 article published in FASEB. The NIH-supported study was among the first to report that synonymous (silent) changes in DNA traditionally considered neutral may adversely affect the processing speed and efficiency of ion channels associated with the heart arrhythmia syndrome Long QT and alter disease severity.
Dr. McDonald has served on multiple study sections of the NIH and AHA. He was elected in 2011 as an AHA Fellow-Basic Cardiovascular Sciences Council.

Dr. McDonald says his clinical practice helps inform and complement the translational science he conducts in the laboratory.
Some things you may not know about Dr. McDonald
- During high school, he worked one year as a head cook for a restaurant in Winter Park, Fla., before entering college. “It made me realize that hard work is important, but also motivated me to study so I could make a living by using my head more than my hands.”
- Wife Kami Kim, MD, also a USF Health physician-scientist, is a professor with joint appointments in the Department of Internal Medicine and in the Department of Global Health. They met in the cardiac intensive care unit at Columbia Presbyterian Medical Center when Dr. McDonald was a resident and Dr. Kim was rounding as a medical student. Their two sons, both studying theoretical math, are Clayton, 24, a PhD student at Boston College, and Vaughan, 20, starting his junior year at Harvard University.
- McDonald enjoys bicycling, Japanese cooking, and nearly exclusively reads fiction – “it’s another window on the human condition.” His two favorite books are One Hundred Years of Solitude, an acclaimed novel by Nobel Prize-winning Latin-American author Gabriel García Márquez, and Infinite Jest, a literary bestseller and unconventional comedy by David Foster Wallace.
-Video and photos by Torie M. Doll, USF Health Communications and Marketing


