USF Health researcher Mack Wu, MD, studies what happens when the microvascular endothelial barrier controlling blood-tissue exchange is compromised during ischemia-reperfusion injury, a condition that can lead to irreversible tissue damage. He also investigates the molecular control of gut permeability, also known as “leaky gut,” in tissue injuries caused by trauma and severe burns.
His group’s work has broad implications for a variety of conditions including stroke, heart attack, thrombosis, sepsis, trauma or other inflammatory diseases associated with microvascular injury.

Mack Wu, MD, is a professor of surgery and molecular medicine at USF Health Morsani College of Medicine and a research physiologist at James A. Haley Veterans’ Hospital. On the monitor next to him are images of microvessels in the small intestine injected with fluorescent dye.
The closely connected endothelial cells lining the interior of blood vessel walls play a critical role in limiting the how much fluid, proteins and small molecules cross the wall of the tiny blood vessels, or microvessels. However when this protective endothelial barrier is damaged, excessive amounts of blood fluid, proteins and molecules leak outside the microvessels into nearby body tissue – a process known as microvascular hyperpermeability. If this breech of endothelial barrier is associated with a body-wide inflammatory response, it can trigger a chain of events leading to edema (swelling), shock from severe blood and fluid loss (hypovolemic shock), and ultimately multiple organ failure.
Pinpointing potential solutions for ischemia-reperfusion injury
Previous research by Dr. Wu’s laboratory and other groups discovered that ischemia-reperfusion injury can cause endothelial barrier damage leading to vascular hyperpermeability, or abnormally leaky blood vessels.
Ischemia-reperfusion injury is typically associated with conditions like organ transplantation, stroke, heart attack, or cardiopulmonary bypass where blood supply to a vital organ is temporarily cut off (ischemia), resulting in oxygen deprivation. For instance, a period of ischemia occurs while a donor organ is transported to a recipient in the operating room, or when a clot interrupts blood circulation to the brain. When blood supply is re-established with new blood returned to the previously oxygen-deprived area (reperfusion), tissue injury can worsen because the reperfusion itself causes inflammation and oxidative damage rather than restoring normal function. It its severest form, ischemia-reperfusion injury can result in multiple organ failure, or even death.
“I believe endothelial barrier injury is one of the key elements of ischemia-reperfusion injury, so my group is trying to find out which molecule is ultimately responsible for the endothelial barrier damage,” said Dr. Wu, a professor of surgery and molecular medicine at USF Health Morsani College of Medicine and a research physiologist at James A. Haley Veterans’ Hospital.
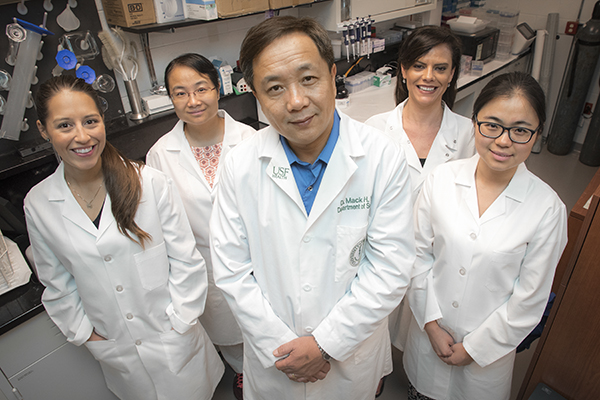
Dr. Wu with some members of his laboratory team. From left, Rebecca Eitnier, research assistant; Shimin Zhang, Department of Molecular Medicine graduate student; Ricci Haines, research associate; and Fang Wang, research assistant.
With the support of a $1.49-million, four-year R01 grant from the National Heart, Lung and Blood Institute, Dr. Wu’s team is zeroing in on a molecule known as focal adhesion kinase, or FAK, an enzyme that may play a role in weakening the microvascular endothelial barrier during ischemia-reperfusion injury. Using cell models and a newly developed mouse model in which the endothelial-specific gene for FAK is knocked out, the USF researchers are testing whether selectively inhibiting FAK activity can rescue the endothelial barrier from such injury.
The work is critical because no FDA-approved treatment exists to prevent tissue damage following reperfusion. Identifying a new mechanism for the injury would provide potential targets for drug development, Dr. Wu said. So for instance, he said, after an initial stroke a new intravenously administered drug selectively targeting endothelial cells in the brain’s microvessels might stop further harmful swelling of the brain caused by stroke.
Defining molecular control of “leaky gut” in severe burn trauma
A second grant from the U.S. Department of Veterans Affairs funds Dr. Wu’s studies to define the underlying molecular mechanisms of leaky guts induced by traumatic injury associated with thermal (fire, scald or chemical) burns. Massive burn trauma is a significant cause of injury and death in American soldiers. With a $960,000 VA Merit Award, Dr. Wu focuses on how intestinal epithelial barrier damage happens during severe burns, with the aim of developing targeted therapies to prevent posttraumatic complications. In particular, he is working to determine the pathways by which the protein palmitoylation in gut epithelial cells are stimulated by burn injury.
Epithelial cells line the interior of the small intestines, and after severe burn injury, this protective epithelial barrier commonly breaks down, causing bacteria and toxins to flow from the intestine into the circulating blood. The result of this abnormal epithelial permeability, or “leaky gut,” can be deadly if sepsis ensues – a bacterial infection in the bloodstream sets up a body-wide inflammatory response leading to multiple organ failure.
While the role gut barrier failure plays in posttraumatic complications is well recognized, its cellular and molecular mechanisms remain poorly understood. Currently, pushing IV fluids to help prevent hypovolemic shock and administering antibiotics and anti-inflammatories are the only therapies, mostly supportive, Dr. Wu said.
“More effective early therapeutic interventions to prevent leaky gut and systemic inflammatory response will be key to preventing sepsis,” he added, whether in soldiers with trauma or VA patients with inflammatory bowel diseases.
From industry to academia
Dr. Wu joined USF Health and the Haley VA Hospital in 2011. He came from Sacramento, Calif, where he was an associate professor of surgery at the University of California at Davis School of Medicine and a research physiologist at Sacramento VA Medical Center. Previously, Dr. Wu was a faculty member in the Department of Medical Physiology at Texas A&M University Health Science Center. He screened pharmaceutical compounds as a toxicologist in a biotechnology laboratory before joining Texas A&M, moving from industry to academia in 1995.
Dr. Wu received his MD degree from Second Military Hospital in Shanghai, China, and conducted an internship at Shanghai Second Hospital.
One of his earliest and most highly cited studies, published in the American Journal of Physiology (1996), was first to report nitric oxide’s role in contributing to cardiovascular injury. The study showed an increase in nitric oxide induces vascular endothelial growth factor (VEGF) to promote leakage in tiny coronary veins.
Another more recent study in Shock (2012) provided direct evidence that thermal burn injury causes intestinal barrier disruption and inflammation characterized by intestinal mucosal permeability (leakage) and an infiltration of immune system cells known as neutrophils.
Something you may not know about Dr. Wu:
He loves deep-sea fishing. Dr. Wu has fished for sharks off the Golf coast of Texas, rockfish off the Pacific coast of California, and grouper off the west coast of Florida.

Dr. Wu is a member of the USF Health Heart Institute. His team’s work has broad implications for a variety of conditions including stroke, heart attack, thrombosis, sepsis, trauma or other inflammatory diseases associated with microvascular injury.
Photos by Eric Younghans, USF Health Communications and Marketing