The founding director of the USF Health Heart Institute has a passion for innovation, translational medicine and entrepreneurship.
Samuel A. Wickline, MD, has parlayed his expertise in harnessing nanotechnology for molecular imaging and targeted treatments into an impressive $1-million portfolio of National Institutes of Health awards, multiple patents and four start-up biotechnology companies.
“We’ve developed nanostructures that can carry drugs or exist as therapeutic agents themselves against various types of inflammatory diseases, including, cancer, cardiovascular disease, arthritis and even infectious diseases like HIV,” said Dr. Wickline, who arrived at USF Health last month from the Washington University School of Medicine in St. Louis.
![]() Dr. Wickline comments on how being a physician adds perspective to the science he conducts.
Dr. Wickline comments on how being a physician adds perspective to the science he conducts.
At Washington University, Dr. Wickline, a cardiologist, most recently was J. Russell Hornsby Professor in Biomedical Sciences and a professor of medicine with additional appointments in biomedical engineering, physics, and cell biology and physiology.
“I like the challenge of building things,” he said.
In St. Louis, he built a 29-year career as an accomplished physician-scientist keenly interested in translating basic science discoveries into practical applications to benefit patients. He served as chief of cardiology at Jewish Hospital, developed one of the first cardiac MRI training and research programs in the country, helped establish Washington University’s first graduate program in biomedical engineering, and led a university consortium that works with academic and industry partners to develop medical applications for nanotechnology.
At USF, there will be no shortage of challenging opportunities to build.
Building the USF Health Heart Institute
A major part of Dr. Wickline’s new job is helping to design, build and equip the Heart Institute. Most importantly, he will staff the state-of-the-art facility with a critical interdisciplinary mix of top biomedical scientists (including immunologists, molecular biologists, cell physiologists and genomics experts), who investigate the root causes of heart and vascular disease with the aim of finding new ways to detect, treat and prevent them. The Heart Institute will be co-located with new Morsani College of Medicine in downtown Tampa; construction on the combined facility is expected to begin later this year.
“I have been impressed by the energy and commitment here at the University of South Florida to invest substantial resources in a heart institute,” Dr. Wickline said. “I believe we have a lot to offer in terms of bench-to-bedside research that could solve some of the major cardiovascular problems” like atherosclerosis or heart failure.
“We want to put together a program that supplies the appropriate core facilities to attract the best and brightest researchers to this cardiovascular institute.”
Cardiovascular disease is the leading cause of death in the United States and worldwide, so exploring potential new treatment options is critical. One of the Heart Institute’s driving themes will be advancing concepts and findings that prove promising in the laboratory into projects commercialized for clinical use, Dr. Wickline said.
“Our goal is to make a difference in the lives of patients,” he said. “Innovation is not just about having a new idea, it’s about having a useful idea.”
Dr. Wickline also serves as associate dean for cardiovascular research and a professor of cardiovascular sciences at the Morsani College of Medicine. He holds the Tampa General Endowed Chair for Cardiovascular Research created last year with a gift from USF’s primary teaching hospital.
With Washington University colleague Hua Pan, PhD, a biomedical engineer and expert in molecular biology, Dr. Wickline is re-building his group at USF. Dr. Pan was recently recruited to USF as an assistant professor of medicine to continue her collaborations with Dr. Wickline.
![]() An example of Dr. Wickline’s group using nanotechnology to help combat atherosclerosis.
An example of Dr. Wickline’s group using nanotechnology to help combat atherosclerosis.

Dr. Wickline’s lab focuses on building nanoparticles to deliver drugs or other therapeutic agents to specific cell types, or targets.
Designing nanoparticles to “kill the messenger”
Dr. Wickline’s lab focuses on building nanoparticles – shaped like spheres or plates, but 10 to 50 times smaller than a red blood cell – to deliver drugs or other therapeutic agents through the bloodstream to specific cell types, or targets. These tiny carrier systems can effectively deliver a sizeable dosage directly to a targeted tissue, yet only require small amounts of the treatment in the circulation to reduce the risk of harmful side effects.
Some types of nanoparticles can carry image-enhancing agents that allow researchers to quantify where the illuminated particles travel, serving as beacons to specific molecules of interest, and enabling one to determine whether a therapeutic agent has penetrated its targeted site, Dr. Wickline said.
Dr. Wickline also is known for designing nanoparticles derived from a component of bee venom called melittin. While bee venom itself is toxic, Dr. Wickline’s laboratory has detoxified the molecule and modified its structure to produce a formula that allows the nanoparticles to carry small interfering (siRNA), also known as “silencing RNA,” or other types of synthetic DNA or RNA strand.
Among other functions, siRNA can be used to inhibit the genes that lead to the production of toxic proteins. Many in the nanotechnology research and development community are working to make siRNA treatment feasible as what Dr. Wickline calls “a message killer,” but the challenges have been daunting.
“The big challenge in the field of siRNA, and many companies have failed at this, is how to get the nanostructure to the cells so that the siRNA can do what it’s supposed – hit its target and kill the messenger — without being destroyed along the way, or having harmful side effects,” Dr. Wickline said. “We figured out how to engineer into a simple peptide all of the complex functionality that allows that to happen.”
![]() Dr. Wickline comments on the underlying similarities between cardiovascular disease and cancer.
Dr. Wickline comments on the underlying similarities between cardiovascular disease and cancer.
Different targets, same delivery vehicle
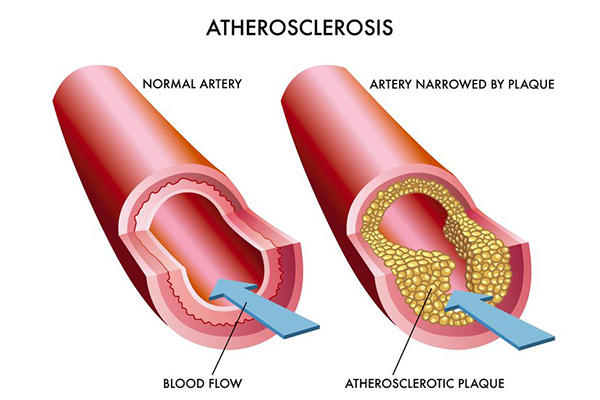
In a recent series of experiments in mice, Dr. Wickline and colleagues have shown that silencing RNA messages delivered by nanoparticle to a specific type of immune cell known as a macrophage – a “big eater” of fat – actually shrinks plaques that accumulate inside the walls of the arteries during atherosclerosis, one of the main causes of cardiovascular disease. The build-up of atherosclerotic plaques with fat-laden macrophages narrows, weakens and hardens arteries, eventually reducing the amount of oxygen-rich blood delivered to vital organs.
This type of plaque-inhibiting nanotherapy could be useful in aggressive forms of atherosclerosis where patients have intractable chest pain or after an acute heart attack or stroke to prevent a secondary cardiac event, Dr. Wickline said.
In another study, Washington University School of Medicine researchers investigated the potential of the siRNA nanoparticle designed by co-investigators Dr. Pan and Dr. Wickline in treating the inflammation that may lead to osteoarthritis, a degenerative joint disease that is a major cause of disability in the aging population. The nanoparticles — injected directly into injured joints in mice to suppress the activity of the molecule NF-κB — reduced local inflammation immediately following injury and reduced the destruction of cartilage. The findings were reported September 2016 in the Proceedings of the National Academy of Sciences.
Previously, Dr. Wickline said, the Washington University group had shown that nanoparticles delivered through the bloodstream inhibited inflammation in a mouse model of rheumatoid arthritis. And, another laboratory at the University of Kentucky is studying whether locally injected siRNA nanoparticles can quell the bacterial inflammation that can lead to a serious gum disease known as periodontitis. Other collaborating labs are using these nanoparticles in pancreatic, colon, and ovarian cancers with good effects.
“The specific targets in these cases may be different, but the nice thing about this kind of delivery system for RNA interference is that the delivery agent itself, the nanostructures, are the same,” Dr. Wickline said. “All we have to do is change out a little bit of the genetic material that targets the messages and we’re set up to go after another disease. So it’s completely modular and nontoxic.”
The St. Louis-based biotechnology company Trasir Therapeutics is developing these peptide-based nanocarriers for silencing RNA to treat diseases with multiple mechanisms of inflammation. Dr. Wickline co-founded the company in 2014 and continues to serve as its chief scientific officer.
Dr. Wickline with colleague Hua Pan, PhD, a biomedical engineer with expertise in molecular biology.
![]() Inhibiting chronic inflammation without getting rid of beneficial immune responses.
Inhibiting chronic inflammation without getting rid of beneficial immune responses.
Calming the destructive cycle of inflammation
Dr. Wickline’s work is supported by several NIH RO1 grants, including one from the National Heart, Lung and Blood Institute to develop and test nanotherapies seeking to interrupt inflammatory signaling molecules and reduce the likelihood of thrombosis in acute cardiovascular syndromes.
In essence, Dr. Wickline said, he is interested in suppressing chronic inflammation, without disrupting the beneficial functions of surveillance by which the immune system recognizes and destroys invading pathogens or potential cancer cells.
“If you can inhibit the ongoing inflammation associated with (inappropriate) immune system response, you inhibit the positive feedback cycle of more inflammation, more plaques, more damage and more danger,” he said. “If you can cool off inflammation by using a message killer that says (to macrophages) ‘don’t come here, don’t eat fat, don’t make a blood clot’ – that’s what we think could be a game changer.”
Another NIH grant has funded collaborative work to develop an image-based nanoparticle that detects where in a compromised blood vessel too much blood clotting (hypercoagulation) occurs, and delivers potent anti-clotting agent only to that site. Formation of abnormal blood clots can trigger a heart attack when a clot blocks an artery that leads to heart muscle, or a stroke when a clot obstructs an artery supplying blood to the brain.
Because this site-specific nanotherapy targets only areas of active clotting, it may provide a safer, more effective approach against cardiac conditions like atrial fibrillation and acute heart attack than existing anticoagulant drugs such as warfarin and newer blood thinners like Xarelto® (rivaroxoban) or Eliquis® (apixiban), all which work systemically and come with raised risk for serious bleeding, Dr. Wickline said.
In a study published last year in the journal Arteriosclerosis, Thrombosis, and Vascular Biology, Dr. Wickline and colleagues found that nanoparticles delivering a potent inhibitor of thrombin, a coagulant protein in blood that plays a role in inflammation, not only reduced clotting risk but also rapidly healed blood vessel endothelial barriers damaged during plaque growth.
The preclinical work showed the experimental treatment “is actually an anti-atherosclerotic drug as well as an anti-clotting drug, so there are many potential applications,” Dr. Wickline said.
Dr. Wickline received his MD degree from the University of Hawaii School of Medicine. He completed a residency in internal medicine, followed by clinical and research fellowships in cardiology at Barnes Hospital and Washington University, where he joined the medical school faculty in 1987.
He has authored more than 300 peer-reviewed papers and holds numerous U.S. patents. Dr. Wickline is a fellow of the American College of Cardiology and the American Heart Association, and a 2014 recipient of the Washington University Chancellor’s Award for Innovation and Entrepreneurship.
– Photos by Sandra C. Roa and Eric Younghans